Turn on Show electron dot diagram. The reason is that electrons are shared in a covalent bond.

Bohr Rutherford Lewis Dot Diagrams Ppt Download
Write an electron configuration for Cd.

. This is the common convention we. Draw an electron dot diagram for each. Write Electron Configuration for an Excited State Exercises Exercise 1.
A step-by-step explanation of how to draw the Se Selenium Se2- Selenide ion Lewis Dot StructureFor the Se and Se 2- structure use the periodic table to. The third electron occupies the next energy level L shell which is a higher energy level than the first and this electron is available to make bonds so this electron is the valence electron. Can paste a pic of your own drawing on paper.
Electron dot diagrams can be used to show the formation of ionic compounds. The valence electrons of an atom are shown in an electron dot diagram. Lewis structures can be made for molecules that contain covalent bonds and for coordination compounds.
Use the Gizmo to create a neutral atom of each of the following elements. H He Li Be B C N O Na Mg Al Si P S F Ne Cl Ar. A beryllium atom with two valence electrons would have the electron dot diagram below.
Electron configurations have the format. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the elements symbol. 100 1 rating Transcribed image text.
Take a nitrogen atom at center. Draw 5 valence electrons around it keep two of them in pairs. The electron dot diagram for helium with two valence electrons is as follows.
Therefore the Li electron configuration will be 1s 2. Draw the electron dot diagram for neutral lithium. How do you draw an electron dot diagram for ammonia.
Li has 1 valence electron. H He Li Be B C N O F Ne Na Mg Al Si 6. By putting the two electrons together on the same side we emphasize the fact that these two electrons are both in the 1 s subshell.
Draw the Lewis dot diagram for a neutral atom of As. H becomes the anion H-. Draw an orbital box diagram for the electrons in a neutral Br atom.
Experts are tested by Chegg as specialists in their subject area. Once we know how many valence electrons there are in Lithi. Now take 3 hydrogen atoms in.
Use the Gizmo to create a neutral atom of each of the following elements. Orbital diagrams use the same basic. Draw the Lewis dot diagram for a neutral atom of As.
To check your work turn onShow electron dot diagram. This is the common convention we. Lithium is the third element with a total of 3 electrons.
Create an electron dot diagram for each of the elements below. Since electrons repel each other the dots for a given atom are distributed evenly around the symbol before they are paired. Usethe Gizmo to help you do this.
The number of dots equals the number of valence electrons in the atom. Each dot represents a valence electron. The first number is the principal quantum number n and the letter represents the value of l angular momentum quantum number.
The number of neutrons for the Bohr diagram of Lithium can be found by subtracting the number of protons. So this all makes the shape of the periodic table look like this. Lithium is neutral and its atomic number is 3 hence the number of protons and electrons available for its Bohr diagram is also 3.
Li becomes the cation Li. H also has 1 valence electron. The electron dot diagram for helium with two valence electrons is as follows.
1 s 2 p 3 d and 4 f for the orbital and the superscript number tells you how many electrons are in that orbital. In writing the electron configuration for lithium the first two electrons will go in the 1s orbital. The Li atom gives away its valence electron to the H atom.
A Brief Tutorial on Drawing Lewis Dot Structures. Draw the electron dot diagram for neutral lithium. 1s 2 2s 2 2p 6.
Write the shorthand notation for Pd. Write the full electron configuration for Pd. This is the common convention we.
By putting the two electrons together on the same side we emphasize the fact that these two electrons are both in the 1 s subshell. Use the Gizmo to create a neutral atom of each of the following elements. Turn off Show electron dot diagram.
We review their content and use your feedback to keep the quality high. By putting the two electrons together on the same side we emphasize the fact that these two electrons are both in the 1 s subshell. For the Li structure use the periodic table to find the total number of valence electrons for Li.
Draw an electron dot diagram for each. The electron dot diagram for helium with two valence electrons is as follows. Turn off Show electron dot diagram.
It is very easy. Lithium which has 3 electrons in total has 2 electrons in the first energy level K shell just like helium but these two electrons are not available for bonding so they are not valence electrons. When you are finished turn on Show electron dot diagram and check your answers.
Draw the Lewis dot diagram for a neutral atom of As. Since 1s can only hold two electrons the remaining electron for Li goes in the 2s orbital. When you are finished turn on Show electron dot diagramand check your answers.
A Lewis electron dot diagram or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Assign quantum numbers to the electrons in the valence shell. The purpose of drawing a Lewis dot structure is to identify the lone electron pairs in molecules to help determine chemical bond formation.

Chapter 7 Objectives 7 1 Determine The Number Of Valence Electrons In A Representative Element Explain How The Octet Rule Applies To Atoms Of Metallic Ppt Download
Solved 4 Diagram Turn On Show Electron Dot Diagram The Valence Electrons Of An Atom Are Shown In An Electron Dot Diagram Each Dot Represents A Course Hero

Bohr Rutherford Diagrams Lewis Dot Diagrams Eve Wongworakul Chemistry Unit
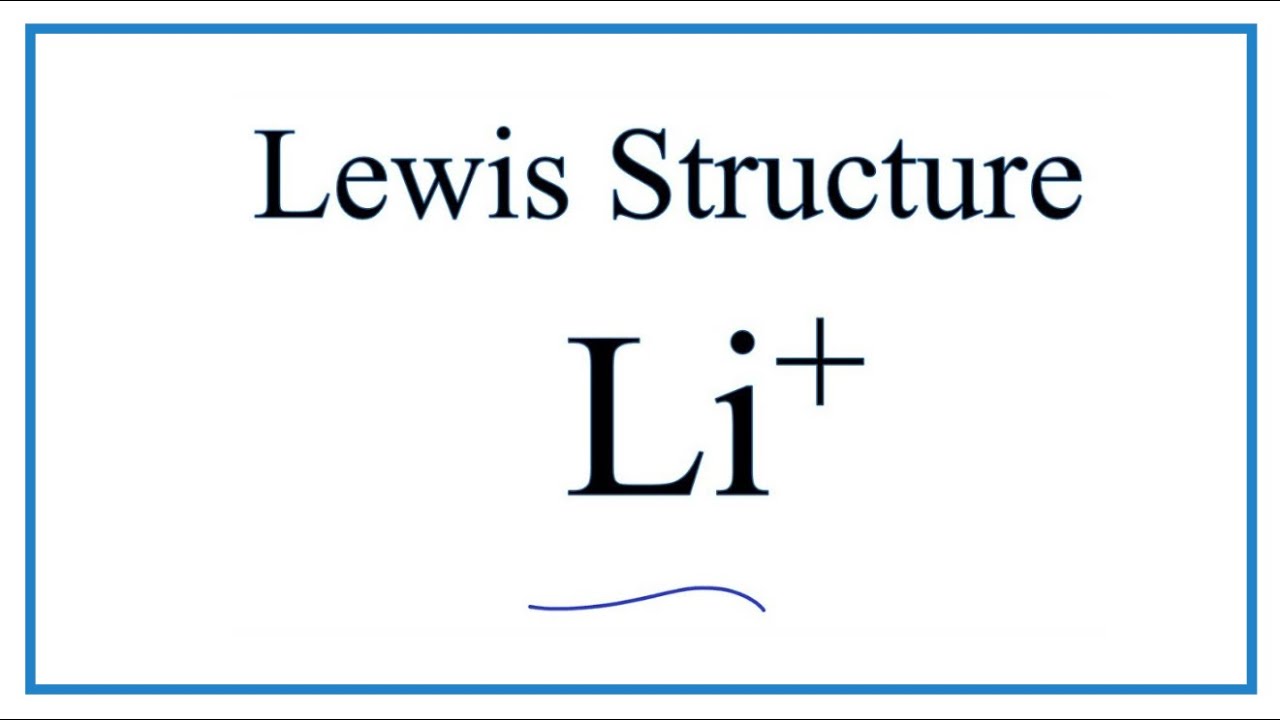
How To Draw The Li Lewis Dot Structure Youtube

Chapters 15 6 Ionic Bonding 15 1 Objectives Use The Periodic Table To Infer The Number Of Valence Electrons In An Atom And Draw Its Electron Dot Lewis Ppt Download

0 comments
Post a Comment